December 01, 2021 | Nora Samaranayake
University of Maryland School of Medicine’s Institute of Human Virology and MitoPower to Initiate Studies
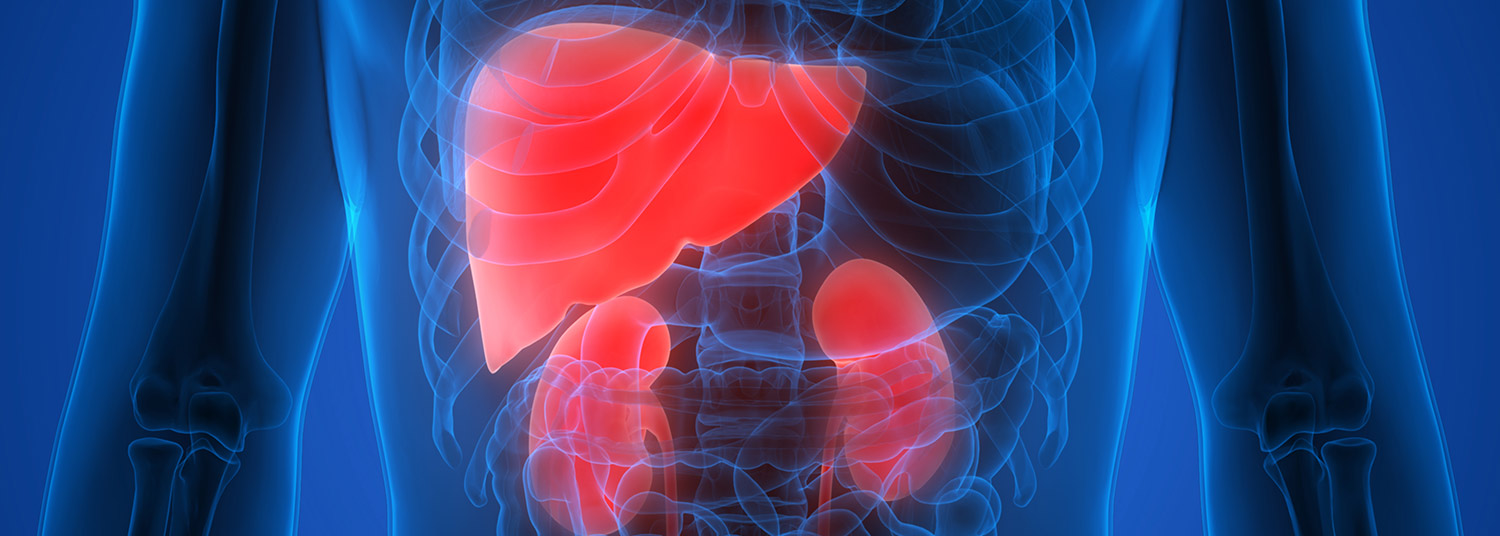
 The Institute of Human Virology (IHV) at the University of Maryland School of Medicine (UMSOM) and MitoPower LLC (“MitoPower”) were awarded an SBIR (Small Business Innovation Research) grant of up to $6.5 million over five years from the National Institute on Alcohol Abuse and Alcoholism. The funds will support the development of MitoPower’s lead compound, MP-04, for the treatment of kidney dysfunction due to alcoholic liver disease, a condition known as alcoholic liver disease-associated hepatorenal syndrome (HRS). The IHV, a Center of Excellence member of the Global Virus Network (GVN), will conduct first-in-human single and multiple ascending dose studies to test the safety of the compound, followed by a Phase 1b study in patients.
The Institute of Human Virology (IHV) at the University of Maryland School of Medicine (UMSOM) and MitoPower LLC (“MitoPower”) were awarded an SBIR (Small Business Innovation Research) grant of up to $6.5 million over five years from the National Institute on Alcohol Abuse and Alcoholism. The funds will support the development of MitoPower’s lead compound, MP-04, for the treatment of kidney dysfunction due to alcoholic liver disease, a condition known as alcoholic liver disease-associated hepatorenal syndrome (HRS). The IHV, a Center of Excellence member of the Global Virus Network (GVN), will conduct first-in-human single and multiple ascending dose studies to test the safety of the compound, followed by a Phase 1b study in patients.
 “There are no current therapeutic options that specifically address the cellular dysfunction and systemic inflammatory response that contribute to the severe impairment of liver and kidney function and progressive organ failure in patients with severe alcoholic hepatitis,” said Mani Subramanian, MD, PhD and CEO of MitoPower. “We are working to complete IND-enabling studies for MP-04 and are excited to collaborate with IHV to characterize this promising compound in human studies.”
“There are no current therapeutic options that specifically address the cellular dysfunction and systemic inflammatory response that contribute to the severe impairment of liver and kidney function and progressive organ failure in patients with severe alcoholic hepatitis,” said Mani Subramanian, MD, PhD and CEO of MitoPower. “We are working to complete IND-enabling studies for MP-04 and are excited to collaborate with IHV to characterize this promising compound in human studies.”
More than 250,000 hospitalizations each year in the U.S. are due to complications of alcoholic liver disease. HRS is an acute complication of cirrhosis (liver scarring) or a severe alcoholic hepatitis (liver inflammation) progressive condition leading to kidney failure. HRS is associated with mortality rates reaching 50%, with many patients requiring invasive treatments such as dialysis and/or liver transplant.
 “There is an urgent, unmet need for an effective therapy to treat HRS caused by severe alcoholic hepatitis and cirrhosis. Globally, the incidence and prevalence of alcoholic liver disease continues to increase and remains a significant cause of liver failure and liver transplantation,” said Prof. Shyam Kottilil, MBBS, PhD, Professor of Medicine and Director of the Division of Clinical Care and Research, Institute of Human Virology at the University of Maryland School of Medicine, and senior advisor to the Global Virus Network (GVN). “MP-04 is a novel therapeutic that has shown promise in preclinical studies to reverse organ dysfunction and systemic inflammatory response syndrome (SIRS) that holds promise in potentially reversing HRS.”
“There is an urgent, unmet need for an effective therapy to treat HRS caused by severe alcoholic hepatitis and cirrhosis. Globally, the incidence and prevalence of alcoholic liver disease continues to increase and remains a significant cause of liver failure and liver transplantation,” said Prof. Shyam Kottilil, MBBS, PhD, Professor of Medicine and Director of the Division of Clinical Care and Research, Institute of Human Virology at the University of Maryland School of Medicine, and senior advisor to the Global Virus Network (GVN). “MP-04 is a novel therapeutic that has shown promise in preclinical studies to reverse organ dysfunction and systemic inflammatory response syndrome (SIRS) that holds promise in potentially reversing HRS.”
The Theodore E. Woodward Chair in Medicine Stephen N. Davis, MBBS, FRCP, FACE, MACP, said, “We are honored to partner academic research with industry to develop therapies that improve our patients’ health and quality of life, especially for chronic diseases for which we have no known treatments.”
 UMSOM Dean E. Albert Reece, MD, PhD, MBA, Executive Vice President for Medical Affairs, UM Baltimore, and the John Z. and Akiko K. Bowers Distinguished Professor, said, “HRS affects many Native American and Alaskan Natives disproportionately, and Black and Mexican Americans are more likely to suffer worse outcomes. Developing an effective treatment will be the first step in finding a way to address these disparities.”
UMSOM Dean E. Albert Reece, MD, PhD, MBA, Executive Vice President for Medical Affairs, UM Baltimore, and the John Z. and Akiko K. Bowers Distinguished Professor, said, “HRS affects many Native American and Alaskan Natives disproportionately, and Black and Mexican Americans are more likely to suffer worse outcomes. Developing an effective treatment will be the first step in finding a way to address these disparities.”
 Robert Gallo, MD, the Homer & Martha Gudelsky Distinguished Professor in Medicine, Co-Founder & Director of the Institute of Human Virology at UMSOM, and Co-Founder and International Scientific Director of the Global Virus Network (GVN), said, “The Institute is pleased to see our Clinical Trials Unit’s portfolio continue to grow under the terrific leadership of Professor Kottilil. While we continue to focus on therapeutics for viruses such as HIV and SARS-CoV-2, it is also important that we research innovations that can combat devastating chronic illnesses, such as liver disease and kidney dysfunction.”
Robert Gallo, MD, the Homer & Martha Gudelsky Distinguished Professor in Medicine, Co-Founder & Director of the Institute of Human Virology at UMSOM, and Co-Founder and International Scientific Director of the Global Virus Network (GVN), said, “The Institute is pleased to see our Clinical Trials Unit’s portfolio continue to grow under the terrific leadership of Professor Kottilil. While we continue to focus on therapeutics for viruses such as HIV and SARS-CoV-2, it is also important that we research innovations that can combat devastating chronic illnesses, such as liver disease and kidney dysfunction.”
This award was granted by the National Institutes of Health under Award Number U44 AA029833. The content of this press release is solely the responsibility of the author and does not necessarily represent the official views of the NIH.
About the Institute of Human Virology
Formed in 1996 as a partnership between the State of Maryland, the City of Baltimore, the University System of Maryland, and the University of Maryland Medical System, the IHV is an institute of the University of Maryland School of Medicine and is home to some of the most globally-recognized and world-renowned experts in all of virology. The IHV combines the disciplines of basic research, epidemiology, and clinical research in a concerted effort to speed the discovery of diagnostics and therapeutics for a wide variety of chronic and deadly viral and immune disorders, most notably HIV, the virus that causes AIDS. For more information, visit ihv.org and follow us on Twitter @IHVmaryland.
About the University of Maryland School of Medicine
Now in its third century, the University of Maryland School of Medicine was chartered in 1807 as the first public medical school in the United States. It continues today as one of the fastest growing, top-tier biomedical research enterprises in the world -- with 46 academic departments, centers, institutes, and programs, and a faculty of more than 3,000 physicians, scientists, and allied health professionals, including members of the National Academy of Medicine and the National Academy of Sciences, and a distinguished two-time winner of the Albert E. Lasker Award in Medical Research. With an operating budget of more than $1.2 billion, the School of Medicine works closely in partnership with the University of Maryland Medical Center and Medical System to provide research-intensive, academic and clinically based care for nearly 2 million patients each year. The School of Medicine has nearly $600 million in extramural funding, with most of its academic departments highly ranked among all medical schools in the nation in research funding. As one of the seven professional schools that make up the University of Maryland, Baltimore campus, the School of Medicine has a total population of nearly 9,000 faculty and staff, including 2,500 students, trainees, residents, and fellows. The combined School of Medicine and Medical System (“University of Maryland Medicine”) has an annual budget of over $6 billion and an economic impact of nearly $20 billion on the state and local community. The School of Medicine, which ranks as the 8th highest among public medical schools in research productivity (according to the Association of American Medical Colleges profile) is an innovator in translational medicine, with 606 active patents and 52 start-up companies. In the latest U.S. News & World Report ranking of the Best Medical Schools, published in 2021, the UM School of Medicine is ranked #9 among the 92 public medical schools in the U.S., and in the top 15 percent (#27) of all 192 public and private U.S. medical schools. The School of Medicine works locally, nationally, and globally, with research and treatment facilities in 36 countries around the world. Visit medschool.umaryland.edu
About MitoPower
Palo Alto, California-based MitoPower is a biopharmaceutical company established in 2017 to develop novel therapies to treat disorders associated with mitochondrial dysfunction. MitoPower maintains numerous academic collaborations in the context of energy metabolism, liver biology and immune function. It has working relationships with world-class CROs in pharmaceutical development, pre-clinical pharmacology, and toxicology. MitoPower’s leadership team has deep experience in drug development in the field of liver diseases, including expertise in medicinal chemistry, pharmacology, toxicology, and regulatory matters. For more information, visit mitopower.com.
Media Contact:
David Murphy
+1-858-774-9052
dm@nventmarketing.com
Contact
Nora Samaranayake
443-823-0613
nsamaranayake@ihv.umaryland.edu
Related stories

Friday, November 17, 2023
Major Funding of Partnership for HIV/AIDS Progress (PFAP) Award from the National Institute of Health (NIH) Office of AIDS Research to the Research Initiative on Infectious Disease and Substance Use (RIIS)
The Institute of Human Virology (IHV) at the University of Maryland School of Medicine (UMSOM) received an annual award for $3 million funded by the NIH Office of AIDS Research. The PFAP award is projected to total approximately $9 million over four years. Principal Investigators are Elana Rosenthal, MD and Sarah Kattakuzhy, MD, MPH.

Tuesday, March 28, 2023
Two-Time Lasker Awardee and Internationally Acclaimed Virologist, Robert C. Gallo, MD, To Step Down as Director of UM School of Medicine’s Institute of Human Virology (IHV)
Robert C. Gallo, MD, one of the world’s leading virologists and cancer researchers, announced he has stepped down from his position as Director of the Institute of Human Virology (IHV) at the University of Maryland School of Medicine (UMSOM), effective March 24.

Monday, October 31, 2022
NCI Grants Awarded to IHV to Prevent Cancer and Improve Screening in Sub-Saharan Africa
Institute of Human Virology (IHV) researchers at the University of Maryland School of Medicine (UMSOM) have received two five-year awards from the National Institutes of Health’s National Cancer Institute (NCI) for a total of $7.5 million. One award aims to reduce the incidence of lung cancer and other cancers associated with using tobacco in Botswana. The other is focusing on improving screening and treatment of anal precancer in Nigeria. Both grants will make use of existing HIV treatment and prevention infrastructure in low- and middle-income countries to reach people living with HIV who are most at risk for these particular types of cancers.

Monday, December 21, 2020
Robert Gallo of the UM School of Medicine Institute of Human Virology and Global Virus Network Awarded Top Life Sciences and Medicine Prize from China
Robert C. Gallo, MD, The Homer & Martha Gudelsky Distinguished Professor in Medicine, co-founder and director of the Institute Human Virology at the University of Maryland School of Medicine and co-founder and international scientific advisor of the Global Virus Network, was awarded the “VCANBIO Award for Biosciences and Medicine,” a significant and authoritative award in the life sciences and medicine field of China. The elite Prize is jointly presented by the University of Chinese Academy of Sciences and the VCANBIO CELL & GENE ENGINEERING CORP, LTD to push forward scientific research, technological innovation and continuous development in the life sciences and medicine field of China.

Tuesday, December 15, 2020
UMSOM Institute of Human Virology’s Shyam Kottilil, MBBS, PhD Receives Top Award from National Physician’s Group
Shyam Kottilil, MBBS, PhD, professor of medicine at the University of Maryland School of Medicine (UMSOM), and Director of UMSOM’s Institute of Human Virology (IHV) Division of Clinical Care and Research, has been awarded Mastership in the American College of Physicians (ACP), the national organization of internists. Dr. Kottilil is also Chief of the Division of Infectious Diseases in the UMSOM Department of Medicine and is a scientific advisory member of the Global Virus Network (GVN).

Monday, October 12, 2020
WJLA (Washington, DC): How long can you spread coronavirus once infected? We found out.
Social distancing, hand hygiene and face masks can help curb the spread of the highly contagious coronavirus but if you do get sick, how long can you spread COVID to others? 7 On Your Side went looking for answers.
Monday, August 31, 2020
UM School of Medicine’s Institute of Human Virology Recruits Top HIV/AIDS Epidemiologist Shenghan Lai Along with Team of Researchers
Robert C. Gallo, MD, the Homer & Martha Gudelsky Distinguished Professor in Medicine, Co-founder and Director of the Institute of Human Virology (IHV) at the University of Maryland School of Medicine (UMSOM), and Man E. Charurat, PhD, MHS, Professor of Medicine, Director of the Division of Epidemiology & Prevention and CIHEB Global Director at the IHV, announced today that Shenghan Lai, MD, MPH and Hong Lai, PhD, MPH, in addition to three staff members, and two more to add, have joined the Institute of Human Virology. The faculty began their positions on April 1 with Professor and Associate Professor academic appointments in the UMSOM’s Department of Epidemiology & Public Health.
Monday, February 24, 2020
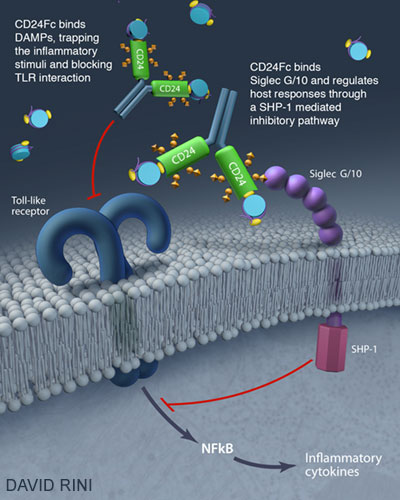
Institute of Human Virology and OncoImmune Launch Clinical Trial to Lessen Effects of HIV Later in Infection
On February 1, the Institute of Human Virology (IHV) at the University of Maryland School of Medicine (UMSOM) and OncoImmune, Inc. launched a new Phase II clinical trial called CALIBER, which will test whether OncoImmune’s lead product, CD24Fc, can reduce late effects of HIV infection, such as inflammatory abnormalities including cardiovascular disease. This is intended for use in HIV patients who have been receiving the traditional antiretroviral therapies that control, but do not cure, HIV infection.

Thursday, March 21, 2019
IHV Experts Researching Experimental Drug to Curb Opioid Cravings
Researchers at the University of Maryland School of Medicine (UMSOM) Institute of Human Virology (IHV) are collaborating with scientists at the National Institutes of Health to test an experimental drug to curb opioid cravings.

Tuesday, December 11, 2018
Institute of Human Virology's Shyam Kottilil to Receive National Award from American College of Physicians
The American College of Physicians announced that Shyam Kottilil, MBBS, PhD, FACP, Professor of Medicine and Director of the Division of Clinical Care and Research at the Institute of Human Virology (IHV) of the University of Maryland School of Medicine (UMSOM) and Chief of the Division of Infectious Diseases, was awarded the American College of Physicians (ACP) Richard and Hinda Rosenthal Award #1 from the Rosenthal Family Foundation.

Tuesday, December 04, 2018
Institute of Human Virology Researchers Discover That a Bacterial Protein Promotes Cancer
The Institute of Human Virology (IHV) at the University of Maryland School of Medicine (UMSOM) announced today the discovery that DnaK, a protein of the bacterium mycoplasma, interferes with the mycoplasma-infected cell’s ability to respond to and repair DNA damage, a known origin of cancer.

Tuesday, October 23, 2018
Institute of Human Virology Hosts 20th Annual International Meeting of Top Medical Virus Researchers in Baltimore, Maryland
The Institute of Human Virology (IHV) at the University of Maryland School of Medicine yesterday commenced IHV’s 20th Annual International Meeting, to be held through Thursday, October, 25 at the Four Seasons Hotel in Baltimore, Maryland. This year, among other viral and cancer related topics, the meeting is holding special sessions on the 40th anniversary of the first human retrovirus, Human T cell Leukemia Virus (HTLV), and the 15th anniversary of the President’s Emergency Plan for AIDS Relief (PEPFAR). IHV’s Annual International Meeting attracts hundreds of elite scientists who descend upon Baltimore to share ideas and inspire medical virus research collaborations.

Wednesday, September 19, 2018
Institute of Human Virology (IHV) Awarded $12M to Combat Opioid Epidemic Through Clinical Research Trials
The Institute of Human Virology (IHV) at the University of Maryland School of Medicine will lead a $12 million dollar project to improve the morbidity and mortality of people with opioid use disorder (OUD). Utilizing a novel compound, IHV researches will implement a series of investigations, entitled SEARCH, to evaluate the underlying mechanisms of craving reduction as a strategy to prevent opioid misuse, dependence, and relapse. The grant is awarded through the National Institutes of Health’s (NIH) Helping to End Addiction Long-term (HEAL) Initiative, made possible through groundbreaking funding from the U.S. Congress.

Thursday, May 03, 2018
UM School of Medicine's Institute of Human Virology Makes Key Appointments to Clinical Care and Research Leadership Positions
Institute Names Dr. Shyam Kottilil and Dr. Anthony Amoroso to replace Dr. Robert Redfield, now Director at CDC.

Wednesday, March 21, 2018
Dr. Robert Redfield, Co-Founder of the Institute of Human Virology at the University of Maryland School of Medicine, to Become CDC Director
The Institute of Human Virology (IHV) at the University of Maryland School of Medicine (UMSOM) congratulates its co-founder and associate director, Robert R. Redfield, MD, on his appointment to be the next director of the U.S. Centers for Disease Control and Prevention (CDC).

Tuesday, March 20, 2018
UMSOM Cancer Expert at Institute of Human Virology Named Fellow of American Society of Clinical Oncology
Clement A. Adebamowo, BM, ChB, ScD, FWACS, FACS, Associate Director of Population Science at the Marlene and Stewart Greenebaum Comprehensive Cancer Center, University of Maryland School of Medicine (UMSOM), and Professor of Epidemiology and Public Health, Institute of Human Virology, has been named a 2018 Fellow of the American Society of Clinical Oncology (ASCO).

Tuesday, November 22, 2016
IHV Awarded $138M to combat HIV/AIDS in Africa & Launches Center for International Health, Education, & Biosecurity
The Institute of Human Virology (IHV) at the University of Maryland School of Medicine announced today more than $138 million in multiple five-year grants awarded by the Centers for Disease Control and Prevention to combat HIV/AIDS in Kenya, Tanzania, Zambia, and Nigeria. The Institute concurrently announced the formation of the IHV Center for International Health, Education, & Biosecurity (CIHEB), and its newly appointed director, Deus Bazira Mubangizi, DrPH, MBA, MPH, Assistant Professor of Medicine, Director, Center for Health, Education, & Biosecurity, Institute of Human Virology, University of Maryland School of Medicine.
Wednesday, April 13, 2016
IHV Releases Data Supporting Community-Based Treatment Providers in Fight Against Hepatitis C
The Institute of Human Virology (IHV) at the University of Maryland School of Medicine released data today at The International Liver Congress 2016 in Barcelona, Spain demonstrating that treatment for hepatitis C virus (HCV) can be provided safely and effectively within a community-based and non-specialist setting.

Tuesday, December 01, 2015
A Statement from the Leadership of the Institute of Human Virology on World AIDS Day
Research enabled treatment to be developed for HIV/AIDS, allowing the Institute of Human Virology (IHV) at the University of Maryland School of Medicine (which Dr. Gallo heads and co-founded with his colleagues Drs. William Blattner and Robert Redfield) to develop a promising HIV vaccine candidate, treat nearly 6,000 patients annually in Baltimore, and care for more than 1 million people in Africa and the Caribbean since 2004.

Tuesday, September 29, 2015
Institute of Human Virology Hosts International Meeting of Prominent AIDS Researchers
The Institute of Human Virology (IHV) at the University of Maryland School of Medicine is hosting IHV’s 17th Annual International Meeting Sunday, September 27 through Wednesday, September 30 at the Baltimore Marriott Waterfront Hotel in Baltimore, Maryland.